Regulatory strategy and quality approach
Regulatory strategy
KLAVA undertakes to take all measures to guarantee the integrity and security of the personal data it will have to process in accordance with the legal and regulatory provisions, in particular the law of January 6, 1978, amended by the law of August 6, 2004, as well as Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 on the protection of individuals with regard to the processing of personal data and on the free movement of such data, and repealing Directive 95/46/EC (General Data Protection Regulation), as of its date of application.
The health data collected in the context of our application development are stored in a certified storage center (HDS). Users are kept informed of the processing of their data and are subject to written consent.
Unlike many health applications, KLAVA Innovation’s mission is to carefully design and validate digital therapies through clinical trials in order to provide clinically validated applications approved by regulatory authorities.

RGPD protection and data security
KLAVA undertakes to take all measures to guarantee the integrity and security of the personal data it will have to process in accordance with the legal and regulatory provisions, in particular the law of January 6, 1978, amended by the law of August 6, 2004, as well as Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 on the protection of individuals with regard to the processing of personal data and on the free movement of such data, and repealing Directive 95/46/EC (General Data Protection Regulation), as of its date of application.
The health data collected in the context of our application development are stored in a certified storage center (HDS). Users are kept informed of the processing of their data and are subject to written consent.
Regulatory strategy
Unlike many health applications, KLAVA Innovation’s mission is to carefully design and validate digital therapies through clinical trials in order to provide clinically validated applications approved by regulatory authorities.

From the development of the app to its wider implementation
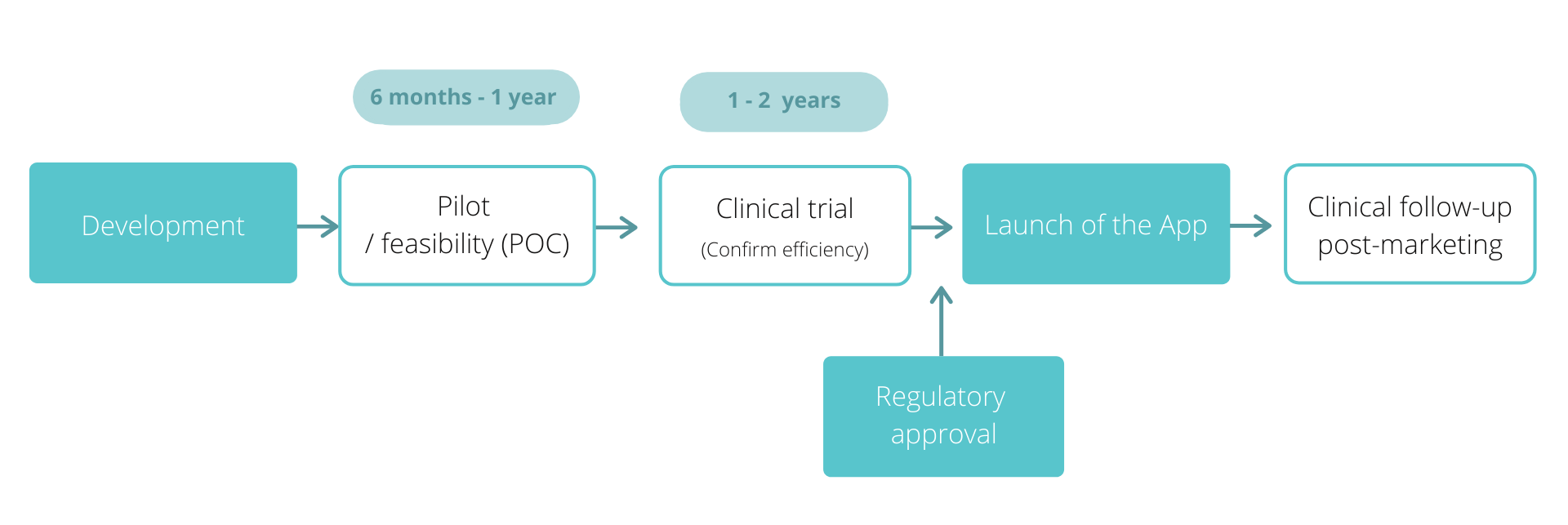
From the development of the application to its wider implementation
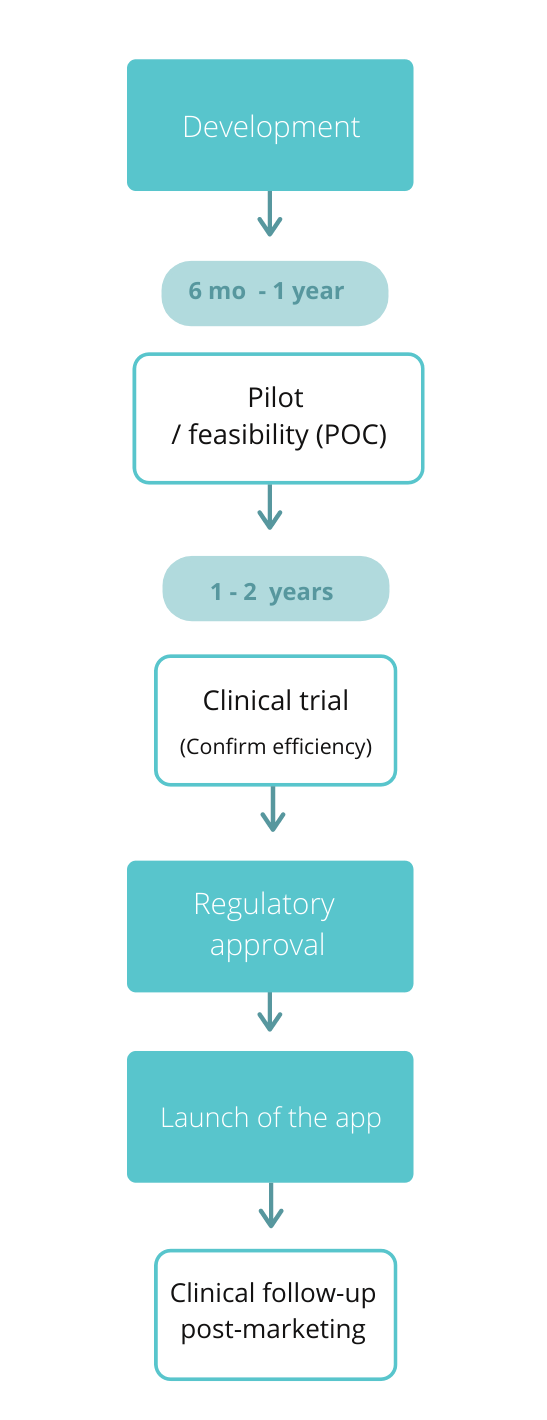

Digital medicine recognised as a medical device

Acquisition of CE Marking


